Promotional Features
Focus on the active all-trans menaquinone MK7 in vitamin K2
The booming market for vitamin K2 as MK7 in western countries and the growing recognition that the vitamin plays a role in bone and cardiovascular health, has made the ingredient more appealing than ever, with numerous players that offer different qualities and origins.
First of all, it is important to highlight that MK7 is the menaquinone that is actively absorbed by the organism and is known to play a role mainly in the extrahepatic tissues in supporting cardiovascular health and bone health. Overall there is no data present in the literature to support a cooperative or synergistic effect of vitamin MK7 with the other menquinones - like MK6, MK8 and MK9 - that are practically inactive.
Gnosis, Italy-based biotechnology company that produces vitamin K2 by fermentation, in its own EU manufacturing plant, is running an information campaign to shed light on the major quality and purity aspects that must be considered to choose the right vitamin K2 ingredient. The most important are the all-trans structure (isomeric purity), the assay purity of MK7 and the impurities profile, which are connected to its biological activity and with the stability of final raw material. Moreover, the different manufacturing processes – by-fermentation or by-synthesis – have to be evaluated because they have a direct impact on the final profile of the ingredient.
Attention to chemical purity, real content of all-trans and of unknown impurities is raising. According to the principle of a close pharmaceutical approach, the more the product is pure, the more it is stable: this is to guarantee quality and suitability in formulations. A pure and stable MK7 ingredient minimizes cross reactions with other ingredients/excipients included in the formulation during the whole shelf of the product.
Since the beginning, the Gnosis project of vitamin K2 development has aimed to achieve these quality and purity features. Gnosis vitamin K2 results in the highest purity (>99%), an all-trans structure (>99%) and almost no impurities. Besides that, the purification technologies implemented meet the market demand for a “more green” ingredient, with no trace of unidentified impurities.
Fermentation-derived MK7: natural-inspired process and expertise benefits from all-trans and purity profile
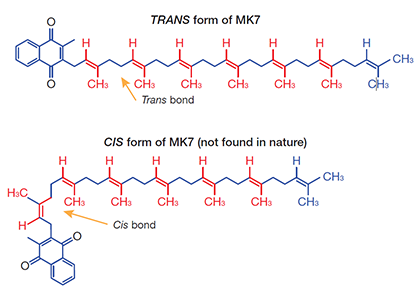
Vitamin K2 as MK7 was isolated for the first time from natto, a traditional fermented soybean food in Japan- Natto is produced via the natural fermentation of boiled soybeans through the action of a specific bacteria, the Bacillus subtilis spp. natto. The biological activity of MK7 is strictly linked to its natural, structural all-trans configuration, such as in natto food. “Trans” identifies the spatial configuration of the aliphatic, isoprenoid side chain double bonds. In humans, the trans isomer of MK7 is essentially responsible for the vitamin’s biological activity and plays a crucial role in bone and cardiovascular health. Cis-analogs of vitamin K2 are biologically inactive.
Gnosis’ industrial manufacturing comes from the well-controlled fermentation processes of a not genetically modified strain of Bacillus subtilis natto, isolated from the commercial Japanese natto food. Since this strain is edible, Gnosis’ fermentation-derived production method offers benefits because it provides an extremely pure and reproducible source of MK7, resembling the characteristics and chemical profile of MK7 – including the all-trans form – found in the naturally-enriched K2 foods.
The resulting vitamin K2 as MK7 - sold under the brand name of vitaMK7® - has been the subject of a patent granted in 2006 (EP 1803820; US 7.718.407; JP 5043425). Gnosis’ process comes from the well-controlled fermentation processes of a non- genetically modified strain of Bacillus subtilis natto, isolated from the commercial Japanese natto food. The strain has been deposited in the DSMZ Collection (Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH), under the accession number DSM 17766.
Gnosis’ bioprocess takes advantage of the same enzymatic pathways of bacteria to execute MK7 production. The process requires the optimization of production medium to maximize the metabolite yield by using a wide range of techniques respecting the natural biosynthetic route of Bacillus subtilis natto. Other key elements of Gnosis’ fermentation-derived process are the optimization of downstream processing for extracting, concentrating and purifying the product from a diluted fermentation broth.
Production process expertise affects vitamin K2 as MK7 quality profile
When working with the different raw materials derived from the same source, it is the quality of the production method that makes the difference.
State-of-the-art biotechnology and expertise of fermentation-derived production processes can critically affect the quality profile of the MK7 ingredient and the all-trans content. In fact, analysis of MK7 finished products present in the market reveals that their isomeric purity is variable and can influence biological responses.
Moreover, very recently, synthetic methods have been proposed for small scale production but the fermentation-derived process from Bacillus subtilis natto is arguably superior in terms of assay of final product, all-trans assay and purity profile. Usually, the synthetic methods produce a mixture of both trans and cis-isomers by default and chemical solvents are used to extract the trans isomer and drive away the cis-isomers and the other impurities. Only the analysis of isomeric purity can unveil the identity and the real content of the menaquinone-7 isomer, discriminating and quantifying the other cis-isomers and regioisomers.
Applying science and know-how on vitamin K2 as MK7 production
With over 20 years of experience in the manufacturing and sales of fermentation-derived ingredients in the nutraceutical and pharmaceutical markets, Gnosis has centered its industrial activity on fermentation-derived ingredients.
The production is performed in a cGMP plant, in compliance with the ISO and HACCP standards, and inspected by the FDA, guaranteeing the origin, quality and safety of all vitaMK7® products. In more than ten years of fermentation-derived MK7 production, Gnosis has been able to respect the natural features of MK7. Organoleptic qualities have been improved through advanced fermentation technologies. The result is a MK7 with the highest purity (>99%), an all-trans structure (>99%) and almost no impurities.
VitaMK7® is free from all known allergens, metals and organic solvents and "Free From" claims can be applied. The manufacturing process does not include starch-containing plant powder like soya or rice flour and is stated as soy- and gluten-free. No solvents are used during the manufacturing process or for the purification steps. VitaMK7® is the only menaquinone-7 in compliance with USP Standard Reference, which defines purity, methodology and requirements of the high-quality standard for an optimal vitamin K2.
References
1 Karl JP, Fu X, Dolnikowski GG, Saltzman E, Booth SL. Quantification of phylloquinone and menaquinones in feces, serum, and food by highperformance liquid chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2014;963:128–33.
2 Bentley R, Meganathan R. Biosynthesis of vitamin K (menaquinone) in bacteria. Microbiol Rev 1982;46:241-2803
3 Conly JM, Stein K. The production of menaquinones (vitamin K-2) by intestinal bacteria and their role in maintaining coagulation homeostasis. Prog Food Nutr Sci 1992;16:307-343
4 Nowicka B, Kruk J. Occurrence, biosynthesis and function of isoprenoid quinones. Biochim Biophys Acta 2010;1797:1587-1605.
5 Knauer TE, Siegfried C, Willingham AE, Matschiner JT. Metabolism and biological activity of cis and trans-phylloquinone in the rat. J Nutrition 1975;105:1519-1524.