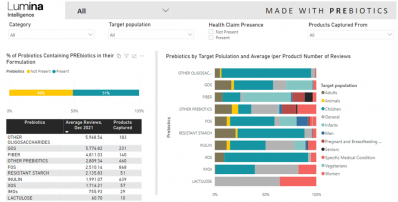
Resveratrol cuts hospitalization rate for COVID outpatients
The new double-blind, placebo-controlled research was published in the journal Scientific Reports. It was the work of researchers associated with a hospital in Ohio and The Ohio State University.
Resveratrol is a polyphenol found in wine grapes, Japanese knotweed and other botanical sources. It was initially studied for its purported ability to mimic the longevity-enhancing effects of ultra low calorie diets.
Resveratrol multi modal action made it a good candidate
The researchers said resveratrol’s combination of health effects made it of particular interest in managing the effect of a COVID-19 infection, which includes the downregulation of ACE2, an important protein involved in the regulation of cardiovascular and renal function.
“Resveratrol’s multimodal antiviral, anti-inflammatory, and antioxidant properties as well as its ability to upregulate ACE2 receptors could be helpful in reducing the clinical effects of COVID-19,” they wrote.
Vitamin D3 (in a single, acute 100,000-IU dose for both treatment and control groups) was included as an adjunct “based upon prior research showing that it has synergistic anti-inflammatory effects.”
The study was conducted under an IND. The researchers recruited 100 patients for the trial via cold calls to patients 45 years old and older who had tested positive at one of the Mount Carmel Health System (Columbus, OH) testing centers.
The primary outcomes for the study were the rate of hospitalization after an initial COVID-19 positive test. At the time the research was conducted in late 2020 the rate of hospitalization among confirmed cases of COVID-19 ranged between 21% in the 45–54 age bracket, up to 31% for patients who were 81 years old or older. The researchers said subsequent analysis showed that actual hospitalization rates were ‘much lower’ though they didn’t specify how much lower.
Additional outcomes included the number of days with fever, breathing problems or fatigue.
In addition to the single megadose of Vitamin D3, the treatment group received a 15-day supply of resveratrol capsules in a 500 mg daily dose. The control group received an identical placebo.
Cutting hospitalization rates
The intervention appeared to cut the rate of hospitalization. One out of the 50-strong treatment group was hospitalized with the 21-day window of the study (a 2% rate), while 3 in the control group required hospitalization (6% rate). In addition, the resveratrol group had 4 emergency room visits and 4 cases of pneumonia, while the control group had 7 ER visits and 8 instances of pneumonia.
“Given the scale of the health and economic impacts of COVID-19, any treatment that can reduce hospitalizations could have a significant impact in this pandemic. RV is generally recognized as safe and has been shown to have positive health benefits in human trials. . . . Given that RV is readily available and could be cheaply scaled up through the fermentation of yeast, it is potentially a scalable solution to treat COVID-19,” the researchers concluded.
Source: Scientific Reports
DOI: 10.1038/s41598-022-13920-9
Randomized double-blind placebo-controlled proof-of-concept trial of resveratrol for outpatient treatment of mild coronavirus disease (COVID-19)
Authors: McCreary MR, Schnell PM, Rhoda DA