Reagan-Udall report calls for sweeping changes in FDA’s food program, leadership structure

The 51-page report, which is at times scathing and highly-critical of FDA’s current approach to food safety management and nutritional guidance, closely examined the agency’s culture, structure and leadership, resources and authorities.
After undertaking a three-phase evaluation protocol that included engaging with the public and stakeholders at a public meeting in late September, reviewing comments and other information gathering, “synthesis and analysis,” an expert panel tapped by the Reagan-Udall Foundation found that “FDA has dedicated staff who are committed to protecting public health,” but “the current culture … is inhibiting its ability to effectively accomplish this goal.”
As such, the panel recommended sweeping changes to the program’s leadership and structure, decision-making process, approach to oversight and enforcement, and how it prioritizes both safety and nutritional guidance.
These conclusions likely come as no surprise to industry and government stakeholders’ whose frustrations with the current scattered approach to food safety and applied nutrition reached a boiling point this summer when an infant formula recall led to massive shortages and prompted FDA commissioner Robert Califf to commission the external review.
In calling for the review, Califf praised FDA food program experts for “significantly [contributing] to the availability of more nutritious food options for consumers,” but noted (in what some might say is an understatement) that “the program has been stressed by the increasing diversity and complexity of the nation’s food systems and supply chain,” as well as by the COVID-19 pandemic, which revealed weaknesses in the agency’s inspections capabilities.
‘Lack of a single clearly identified person to lead’ undercuts program effectiveness
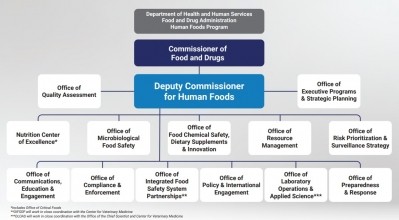
Recognizing that many of their recommendations to strengthen FDA will be difficult and time-consuming to implement, the expert panel repeatedly stresses that positive change must start at the top – with leadership that unites both FDA’s mission to enhance food safety and nutrition and the people currently spread across agency divisions striving to achieve these goals.
“Most FDA employees understand the immense responsibility of the agency’s human foods program, appreciate the importance of their work, and share a common value of striving to protect public health,” the panel intones. “However,” it adds, “the current culture, structure and governance model” that spreads food safety and nutrition oversight across the Office of Food Policy and Response, the Center for Food Safety and Applied Nutrition, and relevant parts of the Office of Regulatory Affairs – all under three different leaders currently – “detract[s] from the program’s effectiveness.”
Without overtly outlining some of the infighting between different FDA food safety and nutrition leaders that has come to light in the past year, the report adds that “lack of a single clearly identified person to lead the Human Foods Program has adversely impacted the organizational culture and led to overlapping roles and competing priorities that result in what is perceived as constant turmoil.”
To amend this and set the tone for productive, positive cultural change, the panel outlined four potential new structures that would bring together the agency’s food safety and nutrition obligations in different ways – helping to create a sense of cohesion and pull ‘food’ out from the long shadow of ‘drug’ with FDA.
Three of these structures include creating a deputy commission for food – a request for which many industry stakeholders, including the Consumer Brands Association, have long lobbied.
“The agency really is in need of that single point that has an expertise in the food arena to make sure that all facets of FDA that are concerned about food safety and nutrition that fall under the umbrella of the foods program are coordinated and talking to one another,” Sarah Gallo, vice president of product policy and CBA told FoodNavigator-USA.
She noted that while restructuring FDA would take time, creating such a leadership position would not require an act of Congress, and could be done quickly by the commissioner.
“The position actually existed during the Obama Administration,” Gallo said. And, while it is unclear why the position was later dissolved, Gallo stressed that it is essential that the person who takes on this role have expertise in food policy and nutrition – something that has not always been true at FDA, which tends to lean more heavily on experts from the medical device and biopharma side.
“Second, we want to make sure that it’s somebody that is culturally right and is going ot take into account a lot of the honest feedback that Reagan-Udall unveiled, and will be ready to work with the agency to put a culture in place that allows for a better working environment that promotes collaboration,” she added.
Finally, she emphasized, the leader must be “truly empowered to oversee all the aspects of the program and have a direct line of authority over” them.
The director for food policy for Consumer Reports echoed Gallo’s sentiment noting that stronger leadership is necessary to “implement a culture of prevention” rather than just one of reaction and recalls, which was long ago called for with the passage of the Food Safety Modernization Act.
Enhanced enforcement authorities, willingness to take ‘precedent setting’ and aggressive action are needed
In addition to empowering a single leader to oversee the food program, the report also advocates for FDA to have additional enforcement priorities and to be restructured so that the policy and enforcement arms of the agency are more closely aligned and can better work together – another point advocated for by CBA and other stakeholders.
Among the additional authorities recommended by the panel is that FDA should be able to disclose non-public information to other agencies with similar and complementary functions so that they can work together more closely on oversight and enforcement.
Similarly, the report argues FDA should be able to request records from food manufacturers in advance or in lieu of an inspection, and be notified when designated food categories are likely to experience shortages or when supply chain disruptions are anticipated – two changes that would allow it to more efficiently allocate resources to areas of need more quickly with the goal of avoiding widespread food safety and public health threats.
The expert panel also urges FDA to use its mandatory recall authority more frequently, and enhance the structure and oversight of the process for designating products as General Recognizable as Safe – two changes that could help remove or keep from distribution harmful products. Likewise, the panel suggest FDA should be able to more easily suspend the registration of food facilities.
Enacting these recommendations and others outlined in the report should help the agency overcome its apparent current adversion to risk, which the expert panel concluded “undercuts its ability to meet its public health mandate.”
It explains that FDA’s Human Foods Program currently is “reluctant to take enforcement action unless they feel that, with certainty, the action could withstand legal challenges.”
While this approach may feel safe, the report argues that true safety comes from a culture that “foster[s] both incremental and far-reaching innovation and encourage responsible and well informed risk-taking,” including in “a willingness to take novel, precedent-setting, and/or aggressive enforcement actions in the name of public health.”
Additional funding, action required
Many of these changes will come at a high price – requiring more staff, additional training and larger budgets for implementation. And while the report does outline some methods for expanding or better using FDA’s food budget, some industry players say it does not go far enough.
Some stakeholders also lamented as insufficient the panel’s suggestions for elevating nutritional guidance – an area that many industry stakeholders have long recognized as getting short shrift from the agency or as being compromised in other ways.
With this in mind, most stakeholders agree that the report is only a first step and that the road to recreating a more deliberate and higher-functioning FDA will be long and likely arduous.