FDA’s update for new Human Foods Program falls short of industry expectations

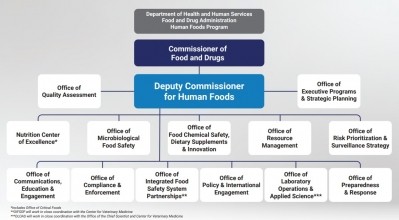
In a job description posted yesterday, FDA declared its intention to hire a deputy commissioner for human food who “reports directly to the Commissioner and serves as a clear line of authority over the entire Human Foods portfolio, which presently includes the Center for Food Safety and Applied Nutrition (CFSAN), the Office of Food Policy & Response, and certain human foods-related components of the of Office of Regulatory Affairs (ORA),” which “will be realigned into a unified Human Foods Program.”
The posting adds the new position will serve “as the principal advisor to the Commissioner of Food and Drugs in matters relating to policy initiatives that involve human food, including safety, innovation and nutrition programs,” and will be responsible for planning, organizing, directing, staffing, coordinating, controlling and evaluating the agency’s human foods programs, including resource allocation, risk prioritization, and strategy for deployment of inspection and field resources dedicated to the human foods program.
“The agency is focused on identifying a candidate that has the expertise to provide leadership over the FDA’s nutrition and food safety programs (including programs aimed at preventing and responding to chemical, microbial, and other hazards). The ideal candidate will have executive-level and real-world experience sufficient to lead the newly envisioned Human Foods Program and its vast remit,” FDA Commissioner Robert Califf said in a statement.
Will the reorg adequately address fundamental flaws?
The new position and reorganization follows a scathing report by the Reagan-Udall Foundation that found the agency’s current culture, structure and governance inhibits its ability to protect public health. A separate internal review of the agency’s infant formula supply chain response also influenced FDA’s “transformative vision” for a unified Human Foods Program.
The accolades industry first gave FDA when the reorganization was announced quickly were tempered with frustrations that stakeholders say have gone unanswered by the agency, prompting even sharper criticism by a coalition, including the Consumer Brands Association, Consumer Reports, STOP Foodborne Illness, the International Fresh Produce Association and others.
They argue additional details about the reorganization that FDA Commissioner Robert Califf outlined yesterday when he announced the national search for a new Deputy Commissioner for Human Foods do not go far enough and suggest the new deputy commissioner’s authority will be divided and diluted.
“Today the commissioner stated he is determining how to best empower the deputy commissioner, leaders of other programs and the associate commissioner for regulatory affairs. … This suggests that authority will be divided,” which calls into questions how decision-making will be streamlined under the reorganization, said Jennifer McIntyre, the chief food safety and regulatory officer at the International Fresh Produce Association.
Citing nearly 28 years of service at FDA, CBA’s VP of Regulatory and Technical Affairs Roberta Wagner lamented such a “matrix management” approach has not worked for FDA’s food program in the past.
“In fact, it’s no secret this management strategy has stunted successful Food Safety Modernization Act implementation for over a decade,” she said.
And yet, she added, “today the commissioner looked at people like me who have dedicated years of public service to FDA regulatory affairs work and said he’s choosing business as usual, instead of the bold changes we’ve adamantly advocated for that would transform the foods program from one of inaction or indecision.”
She also opined the job description posted today “would set anyone up to fail” and likely will deter certain qualified candidates from applying.
“Commissioner Califf must relinquish direct line authority over all FDA foods program components to an expert leader who can turn things around if the program is properly structured for success,” she said.
‘My vision is focused on a new, agency-wide model where the activities … are better synced’
Likely anticipating pushback from industry stakeholders after meeting with them earlier this month, Califf attempted to soothe concerns about the proposed restructuring when he outlined the agency’s next steps.
Acknowledging “a number of questions about the proposed operational changes for ORA and how these plans will work with changes to the Human Foods Program,” he said, “I cannot stress enough that my vision is focused on a new, agency-wide model where the activities and responsibilities of the regulatory programs and ORA are better synced to improve efficiency and effectiveness with clear decision rights so that everyone knows who has authority.”
Next steps in the reorganization
On that note, he explained FDA currently is addressing specific functions of ORA, CFSAN and OFPR to be unified in a new Office of Integrated Food Safety Systems Partnerships, and it is “analyzing inspection and compliance functions that sit within both ORA and program offices across the agency to determine opportunities to streamline operations and clarify decision-making authority at each step of the inspection process as well as integrate new automation and information technology support.”
FDA also is assessing how to improve risk prioritization to better support public health “given the multitudes of demands and the scarce resources” available, he said.
At the same time, he added, FDA is assessing whether some training functions or roles should be “unified into the Human Foods Program and other product programs” or moved elsewhere.
For example, cosmetics regulation and color certification functions will no longer be housed under CFSAN, but rather the Office of the Chief Scientist.
FDA targets fall for final proposal
Recognizing that a reorganization of this size and import will take time, FDA is targeting the fall for a final proposal, which will include not just the new structure but also the budget and “a detailed mapping and crosswalk of staff from the current to new organization.”
The package will then go to Congress for a 30-day notification period, followed by a formal notice in the Federal Register.