FDA heeds call for unified Human Foods Program under one leader as part of ‘transformative vision’ for food safety, nutrition oversight

“Creating a Human Foods Program under a single leader who reports directly to the Commissioner unifies and elevates the program while removing redundancies, enabling the agency to oversee human food in a more effective and efficient way,” FDA Commissioner of Food and Drugs Robert Califf announced this morning.
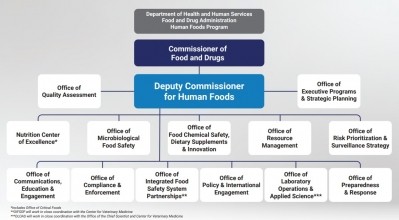
He explained the reimagined program will pull together functions of the Center for Food Safety and Applied Nutrition, Office of Food Policy and Response and certain functions of the agency’s field-based operations under the Office of Regulatory Affairs.
The dramatic overhaul responds to a scathing report by the Reagan-Udall Foundation published last month that found the agency’s current culture, structure and governance inhibits its ability to protect public health, and a separate internal review of the agency’s infant formula supply chain response.
Both reviews were undertaken at the behest of Califf, who said when he commissioned the Reagan-Udall report last July that the agency’s food program “has been stressed by the increasing diversity and complexity of the nation’s food systems and supply chain,” as well as by the COVID-19 pandemic.
However, the problems with FDA’s oversight of food safety and nutrition go back farther than the pandemic, according to current and former agency officials who have long complained that the agency’s oversight of food has suffered in the long shadow of its work on drugs and devices.
In creating a unified Human Foods Program under one leader, Califf said he believes “this proposed approach addresses the recommendations outlined in both reports, and takes into consideration feedback from stakeholders, as well as the voices of employees working in the Human Foods Program who had the opportunity to share input through numerous interactive and listening sessions over the past month.”
As dramatic as the overhaul is—does it go far enough?
The Consumer Brands Association lauded the overhaul and appointment of a deputy commissioner for food, but questioned whether it goes far enough—noting that not all components of the food program will be consolidated in the proposed new structure.
“While today’s announcement is a positive first step, it fails to provide the deputy commissioner with direct line of authority over all major food program components or fully integrate the agency’s policymaker with its inspection force,” CBA’s Vice President Regulatory and Technical Affairs Roberta Wagner said in a statement.
She explained: “We are concerned that anything short of this and a fully empowered deputy commissioner will make it difficult to truly unify the program and deploy the prevention mindset envisioned under the Food Safety Modernization Act.”
CBA and a coalition of diverse industry and consumer stakeholders reiterated last night the need for a unified cohesive organizational structure that included not only human food, but also the Center for Veterinary Medicine and related components of ORA, including inspection and compliance, food-related laboratories, import oversight, state partnerships, training and information technology.
They add that the deputy commissioner should have food safety and nutrition knowledge and expertise, which may sound like a given but has been a rare attribute among FDA leaders, who tend to come from medical and device backgrounds.
Given the extensive silos, in-fighting and frustration caused by a lack of clear leadership in recent years, a “prerequisite to the success” for a deputy commissioner’s structural reform of the agency’s food group should also include a “mandate to modernize the program in a way that facilitates transparency, timeliness and meaningful stakeholder engagement as part of its decision-making process,” said the coalition, which in addition to CBA included American Frozen Food Institute, Association of Food and Drug Officials, Consumer Reports, Environmental Working Group, International Fresh Produce Association, STOP Foodborne Illness and Western Growers.
Finally, the coalition argues the deputy commissioner must be empowered to represent food program issues beyond the agency to Congress, within the executive brand and foreign partners.
“The FDA’s foods program is a distinct part of the agency and deserves a structure and leadership model that is appropriately customized to fit its mission,” they concluded, emphasizing their ongoing commitment to work with FDA to implement changes.
Wagner added this morning that CBA encourages Califf “to be bold and continue exploring durable solutions that will enable the agency to move at the speed of the consumer to ensure they have access to the essential goods they rely on."
“The commissioner’s sustained empowerment and support will be critical as the new deputy commissioner pursues accountability and oversight of many critical functions.”
As FDA launches a “competitive national search” for the deputy commissioner for Human Foods, Califf said the agency will look for someone who can lead the newly unified program to keep “the foods we regulate safe and nutritious, while ensuring the agency remains on the cutting edge of the latest advancements in science, technology and nutrition.”
He added that the deputy commissioner will have decision-making authority over “policy, strategy and regulatory program activities within the Human Foods Program, as well as resource allocation and risk-prioritization.”
A closer look at the new structure
In addition to pulling together under one leader all aspects of human food, Califf’s new vision for the FDA Human Foods Program will include the creation of a Center for Excellence in Nutrition that will be tasked with helping consumers make more informed food choices and helping industry offer healthier options. Within this center will be an Office of Critical Foods called for in the 2023 Consolidation Appropriations Act.
An Office of Integrated Food Safety System Partnerships also will be established to help coordinate the agency’s work with state and local regulatory partners “to more effectively meet the vision … in the FDA Food Safety Modernization Act of 2011,” Califf said.
A Human Foods Advisory Committee of external experts also will created to advise FDA on food safety, nutrition and innovative food technology issues.
Across each of these elements, Califf promises to “strengthen our enterprise information technology and analytical capabilities to fulfil the promise described in the New Era of Smarter Food Safety.”
The Center for Veterinary Medicine will remain outside of the new Human Foods Program, despite calls for its inclusion, Califf said “relevant food safety activities will be closely coordinated between the CVM Center Director and Deputy Commissioner for Human Foods.”
The elements of ORA that are not folded into the new Human Foods Program will be “transformed into an enterprise-wide organization that supports the Human Foods Program and all other FDA regulatory programs,” Califf said, adding: “this realignment will allow ORA to be singularly focused on excellence in its core mission—inspections, laboratory testing, import, and investigative operations.”
Next steps
Change of this magnitude will not happen overnight and while FDA works out the kinks, CFSAN, ORA and OFPR will continue to operate as is, Califf said.
To help hammer out the details of the vision announced today and ensure its successful execution, Calif added FDA created an Implementation and Change Management Group. He also promised to keep stakeholders and the public in the loop with the next update coming end of February.