Promotional Features
What to expect when conducting a dietary supplement clinical trial
Clinical research can unlock incredible market opportunities for dietary supplements. Companies use clinical trial data to gain access to new markets, support unique claims or explore new regulatory pathways.
The problem is, clinical trials can be daunting. Companies large and small struggle to find the right trial design, recruit enough subjects and meet budget and timeline goals.
Here we provide an overview of the clinical trial process for dietary supplements so that you know what to expect when conducting research for your product. We also address common reasons for clinical trial delays and how you can mitigate these risks.
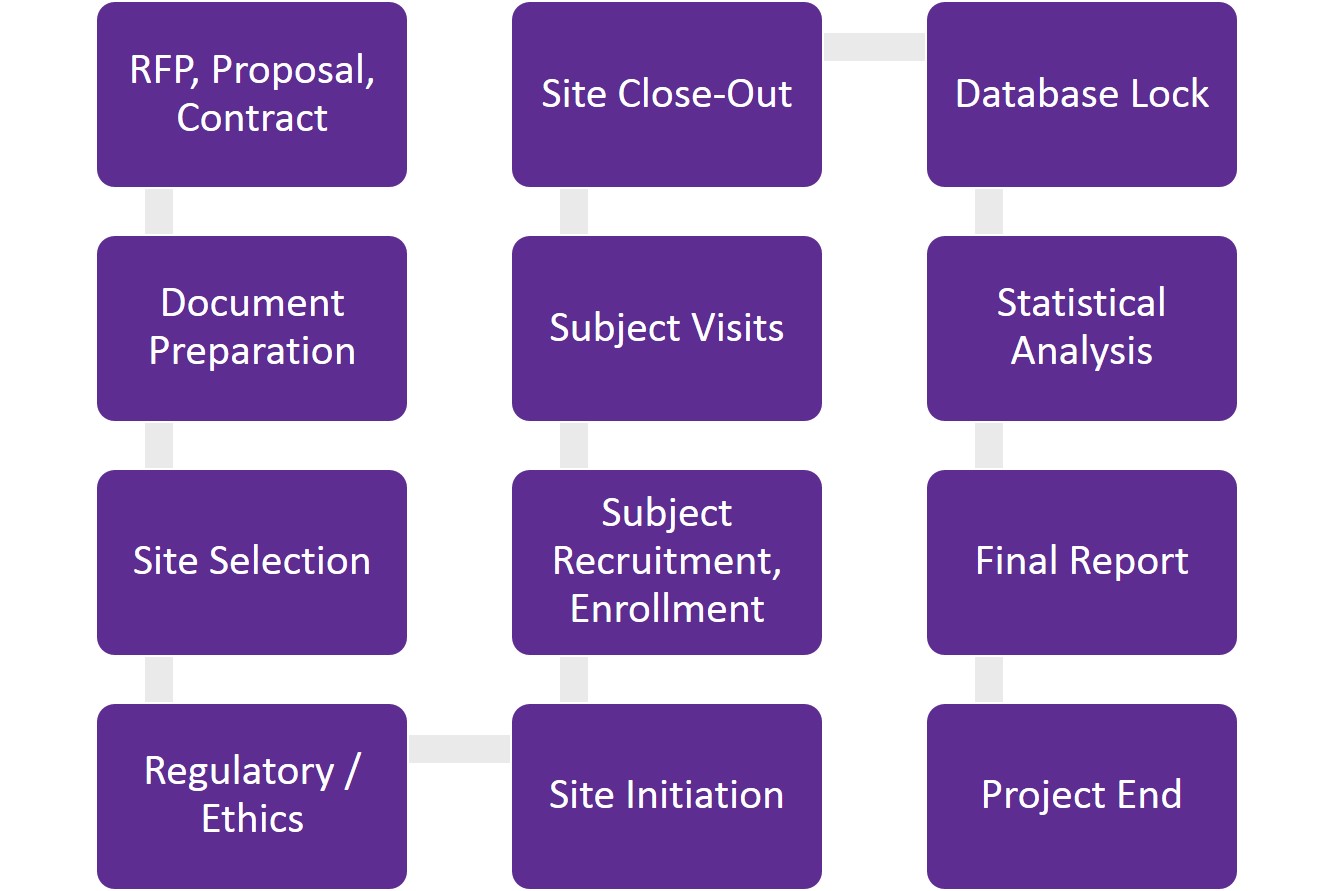
Understanding clinical trial milestones
The proposal – setting goals and timelines
If you are working with a contract research organization (CRO) to conduct your clinical trial, the first step is usually a request for proposal from you, the sponsor. After determining study objectives, endpoints, duration and estimated number of subjects, the CRO provides the sponsor with a proposal. A good proposal is detailed and transparent, providing full visibility on what is included — and not included — in the cost. Revisions at this stage are the norm. After the proposal is accepted, a clinical trial agreement is drafted and reviewed by both organizations’ legal teams. Once the clinical trial agreement is signed, the project can begin.
The start-up period – planning and site selection
The start-up phase of a clinical trial is when all the planning takes place. It is a very labour-intensive period that takes time due to the numerous documents being written, reviewed and approved.
During this stage, the protocol is written and finalized, site selection is underway and ethics and regulatory (if applicable) submissions are completed. Regulatory review is an additional step applicable to the Canadian market. It may seem unnecessary and time-consuming but is critical in mitigating risks. The Canadian regulatory authorities review the study’s scientific rigour, merit and safety/ethics for subjects – all of which are advantageous for studies intended to substantiate product claims or obtain regulatory approval.
Site selection is an important step in the start-up period. Choosing the right site (or sites) for your clinical trial requires careful consideration of timelines and budget. Will it be a single-centre or multi-centre study? What are the subject population inclusion/exclusion criteria? How many subjects are needed per month? These are just some of the questions that need to be answered as part of clinical trial site selection.
Study start-up averages three months, but in Canada requires an additional two to three months for regulatory review and approval. If the study is conducted in the US or is a food-based rather than dietary supplement study, two weeks to one month is usually required for ethics approval.
The study period – site initiation, subject recruitment and subject retention
Site initiation is the green light for the study to start screening subjects. Getting the study up and running starts with the study monitor visiting the site, ensuring product has been sent to the site, and all approvals are in place prior to initiating.
Subject recruitment is often the single largest reason for a delayed clinical trial. In fact, most studies fail to meet recruitment timeline goals. Recruitment is not a linear process. It typically follows a bell curve, as it takes time for a campaign to gain traction. Thus, early recruitment is slower than later stages.
It is also important to understand that only about 3% of the general adult population is willing to take part in a clinical trial and only a small subset of those will be eligible to screen. Even fewer will meet the enrollment criteria. It is vital that your CRO or research partner effectively manage recruitment so that you can execute your study quickly and efficiently. If a study is particularly difficult to recruit for, a multi-centre trial may be advantageous.
During the study period, subjects are screened, eligible subjects are enrolled and subject visits begin. Not all subjects will complete a study. Withdrawals occur for various reasons. Research teams that communicate effectively, provide good customer service and achieve quality care help ensure subject compliance and retention. Additional factors include having a protocol that is easy to comply with, as well as fair compensation for subjects. Recruitment continues until the last subject’s last visit has taken place, at which time the study monitor will conduct a close-out visit at the site. This is the official end of the study period phase. Data entry and monitoring are ongoing through this phase.
Project end – data management and statistical analysis
Once all data is entered, reviewed and checked, the database can be locked. This usually happens within days of the close-out visit. The locked database is then transferred to the statistician for analysis and a final report is produced. The review process can vary depending on the CRO and sponsor’s review timelines. Generally, it takes 6 to 8 weeks from starting statistical analysis to finalizing the statistical output, followed by approximately 8 to 12 weeks to write and edit the report after one round of revisions.
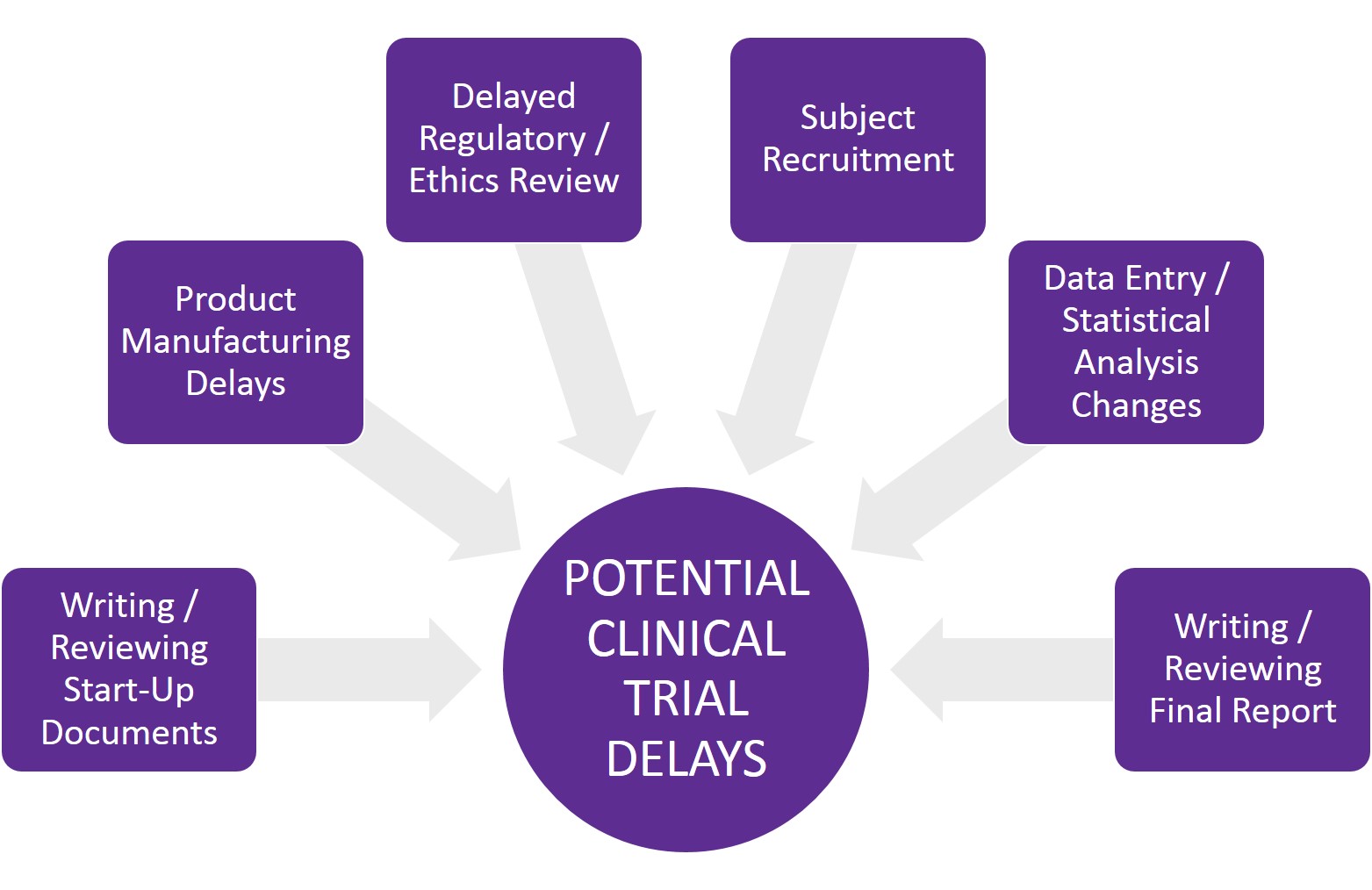
Anticipating potential issues that could delay your trial
Several factors can delay a clinical trial at any point during a clinical project. Some are within the sponsor’s control, while others result from third-party vendors or regulatory agency delays that neither the sponsor nor CRO can control.
The most common reasons for clinical trial delays include:
- Turnaround time for writing, reviewing and editing start-up documents – CRO, sponsor or third party
- Product being studied is not ready – sponsor or third party
- Delayed review of regulatory and/or ethics documentation – third party
- Recruitment problems – CRO, sponsor or third party
- Testing delays (if biological endpoints are included in the study) – third party
- Data entry and clean-up takes longer than anticipated – CRO or third party
- Statistical analysis delays or changes – third party
- Writing, reviewing and editing the final report – CRO or sponsor
- Project scope changes – CRO, sponsor or third party
Knowing what potential issues may arise and having strategies in place to deal with them is crucial to overcoming these barriers. Communicating effectively and in a timely manner is also essential so that all parties are aware of the project’s progress.
Achieving your clinical trial objectives
A clinical trial is a complex project with many moving parts. Planning and executing a clinical trial that achieves your project goals requires strong management and strategic oversight.
Partnering with a CRO that understands the importance of effective planning, budgetary and timeline considerations and maintaining open communication and collaboration with all partners — the sponsor, site, labs, vendors, regulatory agencies and ethics boards — is mission critical.
Above all, balancing scope, costs and timelines is required to ensure high-quality results – the essential piece you need to substantiate your product claims with confidence.
----
Nutrasource is a full-solution CRO that helps companies support product claims to gain market access. With over 100 staff across four sites in North America, our experienced team provides you with the clinical trial expertise you need to launch your dietary supplement with strong science and regulatory confidence.